Effect of ResQFoam to Slow or Stop Severe Internal Bleeding in Trauma Patients
Objetivo
Trauma is the leading cause of death in people younger than 45 years. Unfortunately, 40-50% of severely injured patients with internal bleeding die, even after reaching the hospital. This clinical study is about whether ResQFoam can help slow or stop severe internal bleeding in the abdomen (belly area) while the patient is being brought in for surgery. ResQFoam is a device that injects a liquid foam into the abdomen. The foam expands and forms a seal around the bleeding wounds, reducing blood loss.
Palabras clave: Hemorrhagic Shock, Trauma, Severe Bleeding, Internal Bleeding, Traumatic Shock, REVIVE study, ResQFoam, Hemorrhage Control
Sitios de estudio
LAC+USC Medical Center, 2051 Marengo St, Los Angeles, CA 90033
- Men & Women
Lo sentimos, pero este ensayo ya no está inscribiendo voluntarios.
¿Qué hay involucrado?
-
30 days
-
1 (hospital stay, up to 30 days)
-
None
-
ResQFoam inserted in Emergency Room
-
Foam surgically removed in Operating Room
-
Compensation is not available for this study.
-
Study-related procedures are covered.
Elegibilidad
Criterios de inclusión
- 15 years old or older
- Exsanguinating hemorrhage from abdominal source
- Traumatic blunt injury
Criterios de exclusión
- Non-survivable brain injury
- CPR > 5 min prior to ED arrival
- Burns > 20%
- Known DNR
- Known or suspected pregnancy
About This Study
What is Trauma?
Trauma is a physical injury usually caused by violence or road traffic accidents. Trauma is the leading cause of death in people younger than 45 years. Trauma patients may have extensive internal bleeding. People who suffer severe trauma usually need emergency care to stop bleeding. Internal bleeding in the abdomen (belly area) is a difficult problem. In most patients who are severely injured, the only way to stop the internal bleeding in the abdomen is by major emergency surgery. Almost half of the patients who suffer from abdominal bleeding die even after they reach the hospital because they bled too much. There is an urgent need for new ways to treat patients with bleeding in the abdomen.
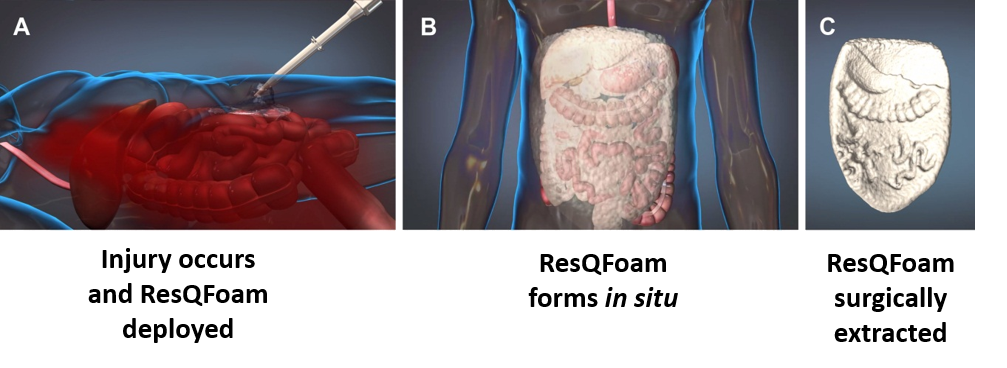
A company called Arsenal Medical, has developed a product called ResQFoam to temporarily slow or stop internal bleeding in the abdomen. ResQFoam has been shown to be effective in animals but has not been tested in people. ResQFoam has not been approved by the Food and Drug Administration (FDA) and so is called an investigational device. The purpose of this clinical study is to study whether ResQFoam can help slow or stop severe internal bleeding in the abdomen while the patient is being brought in for surgery.
This research study is funded by Arsenal Medical and Department of Defense.
What is Exception from Informed Consent (EFIC)?
EFIC is a FDA rule that allows research studies in certain emergency situations to be conducted without consent.
EFIC can only be used when:
- The person’s life is at risk, AND, the treatments we have don’t work, AND, the study might help the person, AND
- It is not possible to get permission from the person because of his or her medical condition NOR from the person’s guardian because there is a very short amount of time required to treat the medical problem.
Before researchers may do a study using EFIC, they must provide information about the study to the community and get their feedback. The FDA has given permission for this clinical study to be done. This clinical study in which patients with severe internal bleeding may be included in the study without their prior consent.
Why is it not possible to get patient’s consent before including them in the study?
People who have had severe internal bleeding are usually in shock due to the blood loss. The heavy loss of blood usually makes people feel confused and they are not in a state to give their consent to take part in a research study. In the majority of trauma cases, the injured person does not have any family nearby to give permission to participate on behalf of the injured person. ResQFoam must be used as soon as possible to help to slow or stop severe internal bleeding in the abdomen. The study doctors and study staff will make attempts to reach family before enrollment. If patients are enrolled into the study before consent was obtained, the study doctors and study staff will talk with the patient and/or family as soon as possible after the procedure to obtain consent for continued participation in the research.
Who will be included in this research study?
Trauma victims who have had severe internal bleeding in the abdomen who need immediate surgery and may not survive transportation to the operating room will be included in this research study. We plan to include people who are at least 15 years old.
What are the study procedures?
When a trauma patient arrives in the emergency department the emergency doctors will examine the patient and decide if they meet the criteria for being in this research study. If the doctors confirm that the patient is in shock due to severe bleeding in the abdomen and is likely to die without emergency surgery, the doctors will deliver ResQForm into the abdomen through a tube. The patient will then be immediately transported to the operating room. In the operating room, the foam will be removed and the patient will be given usual care for treatment of the injuries. Information about the injury and care given until discharge from the hospital will be collected for the research study.
Figure 1 - Foam Schematic
How many patients will be included in this clinical study?
We plan to include up to 40 trauma patients at 4 to 5 hospitals around the country. At LAC+USC, we plan to include about 8 people in this clinical study.
If I do not wish to be included in this clinical study, how can I make my wish known?
If you do not wish to participate in this clinical study, please contact us by filling out the "I am Interested" section at the top of this page, or send us your information through the survey link provided at the top and bottom of this site. We will be happy to provide you with an ‘opt-out’ bracelet that you should wear for the duration of the study. You can also update and carry your Advance Medical Directive. You can also let your family know your wishes.
If you are in the study and decide you no longer want to participate, you can withdraw from the study by notifying the study team in person, in writing or by calling.
The Risks
The possible risks of using ResQFoam are delay in getting to surgery, reaction to the chemicals in the foam, and infection or damage to the organs in the abdomen.
There is also a risk of carbon dioxide accidentally injected into the blood vessels which may cause blockage of the blood vessel. There is a small risk that patients who did not need abdominal surgery for their injury will now need surgery to remove the foam.
Feedback Survey
Thank you for your time. Please take the 9-question survey to help us better understand your thoughts and feelings about this study.
Survey link: https://is.gd/REVIVEStudy
The survey will collect your knowledge about the study and how you feel about this study. At the end of the survey, you can write in your comments, concerns and questions, and provide contact information.
Equipo del Programa
For questions about this study, contact:
- Trauma Research Program
- (323) 205-5503
- trauma@med.usc.edu
We respect your privacy!
Toda la información que usted nos dé será guardada en una base de datos segura y protegida. Toda la información que usted decida compartir se mantendrá de manera confidencial y privada. Lea la Política de Privacidad de la universidad University of Southern California aquí.